Warning: Illegal string offset 'source_type' in /home/mychutej/public_html/blog/wp-content/plugins/egany-facebook-to-wp/egany_facebook_to_wordpress.php on line 1099
The worst known outbreak of the meningitis epidemic inSub-Saharan Africa occurred in Northern Nigeria in 1996. Cases were in excess of 100,000, disproportionately affecting children, and left over 12,000 peopledead.
In the wake of this unprecedented epidemic, Pfizer, one ofthe world’s largest pharmaceuticals arrived in Kano in April 1996, to conductclinical trials for its new antibiotic- Trovan. It was believed by the Pharmaceutical giants that Trovan was a more potent antibiotic to be used against infectious diseases such as Meningitis. In 2 weeks, the trials were over and the team was gone. However, the impact of those weeks were far from being over. In the 200-patient drug trial, a total of 11 children died with many more suffering permanent disabilities most notable of which was brain damage.
In the aftermath of this, there have been huge suspicions of foreign drugs- notably Vaccines in Northern Nigeria as the Pfizer experience had left many wary of foreign aid programmes. Even nearly 10 years after, Kano suspended immunization for 10 months in 2005 over suspicions by religious leaders that the Polio vaccine was laced with harmful substances which may cause sterilization, HIV and consequently death. The government allowed Polio vaccination to resume only after independent tests were conducted and vaccines had to be ordered from Indonesia, a Muslim country.
The international declaration of Helsinki details extensively, the criteria to be met with respect to carrying out clinical trials with humans. In demanding that at all times, the physician acts in the best interest of the patient, strict requirements such as informed consent, ethical approval and conduct of research only if the importance of the objective outweighs the risk and burdens to the subject are fully detailed in the declaration to prevent any form of exploitation. In line with this, the 10-point Nuremberg code further buttresses the regulations in human subject research.
Clinical trials involving new drugs are required to go through 4 phases, 0,1,2,3 before being approved for use in the general population. Each stage of the drug trial is regarded as an independent study and gives unique information about the drug. Drugs are generally considered safe enough for public use after passing through phase 3 of a clinical trial where it is tested on large groups of people to determine efficacy, monitor side effects and compare it to existing treatments. Therefore, concerns with regards to using trial drugs stem from limited knowledge of both short term and long term effects of the drug, which may include harmful genetic mutations, permanent brain damage and even death.
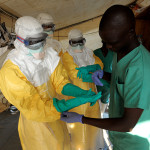
In determining the use of an experimental drug in the wake of an epidemic such as Ebola, a thorough risk-benefit analysis is conducted and experimental drug use is only considered when it remains the best available option in patient management. A clear example of this may be seen in the use of the experimental drug ZMAPP in the United States in the management of its first Ebola cases. Expedited clinical trials may also be applied in the wake of an epidemic to hasten the various phases of drug trials. With Ebola raging on, various experimental drugs have been proposed. Research and development of vaccines is also being
Sped up by large pharmaceutical corporations to combat the epidemic. It is thus at a time like this that thorough respect for due process must be ensured. Sufficient animal testing must be carried out before any trial is done with the Africa population. Risk-benefit ratio for the individual subjects and the implications general good of the society must be thoroughly scrutinized. Ethical approval must be obtained from the Research and Ethical committees existing in the various countries where drug trials may be proposed. Local scientists should be carried along in research design and implementation and knowledge sharing must be the norm.
Good will is just not good enough. If we are to avoid a re-enactment of the awry Kano experience in West Africa, we cannot afford to cut corners in a quest to release a drug in record time or to seemingly halt an epidemic.
Nnewuihe Obinna.












April 19, 2015 at 9:43 am
Hi my name is Janette and I just wanted to drop you a quick note here instead of calling you. I came to your Using Untested Drugs On Africans | EbolaAlert Blog page and noticed you could have a lot more traffic. I have found that the key to running a successful website is making sure the visitors you are getting are interested in your subject matter. There is a company that you can get targeted traffic from and they let you try their service for free for 7 days. I managed to get over 300 targeted visitors to day to my site. Visit them here: http://hud.sn/702k
April 22, 2015 at 1:40 pm
Hi, my name is Victoire and I am the sales manager at SwingSEO Solutions. I was just looking at your Using Untested Drugs On Africans | EbolaAlert Blog website and see that your website has the potential to get a lot of visitors. I just want to tell you, In case you don’t already know… There is a website network which already has more than 16 million users, and the majority of the users are interested in topics like yours. By getting your website on this network you have a chance to get your site more visitors than you can imagine. It is free to sign up and you can read more about it here: http://ft1.info/8ogz – Now, let me ask you… Do you need your site to be successful to maintain your way of life? Do you need targeted traffic who are interested in the services and products you offer? Are looking for exposure, to increase sales, and to quickly develop awareness for your website? If your answer is YES, you can achieve these things only if you get your website on the service I am talking about. This traffic service advertises you to thousands, while also giving you a chance to test the service before paying anything. All the popular sites are using this network to boost their readership and ad revenue! Why aren’t you? And what is better than traffic? It’s recurring traffic! That’s how running a successful site works… Here’s to your success! Read more here: http://claimyourexcellence.info/24e – or to unsubscribe please go here: http://bbqr.me/n1
May 4, 2015 at 10:17 am
After getting more than 10000 visitors/day to my website I thought your blog.ebolaalert.org website also need unstoppable flow of traffic…
Use this BRAND NEW software and get all the traffic for your website you will ever need …
= = > > http://autopilot-mass-traffic.com/download.html
In testing phase it generated 867,981 visitors and $540,340.
Then another $86,299.13 in 90 days to be exact. That’s $958.88 a
day!!
And all it took was 10 minutes to set up and run.
But how does it work??
You just configure the system, click the mouse button a few
times, activate the software, copy and paste a few links and
you’re done!!
Click the link BELOW as you’re about to witness a software that
could be a MAJOR turning point to your success.
= = > > http://autopilot-mass-traffic.com/download.html
Best regards
Your friend
Patricia
May 8, 2015 at 1:33 pm
Hi my name is Olivie and I just wanted to drop you a quick note here instead of calling you. I came to your Using Untested Drugs On Africans | EbolaAlert Blog page and noticed you could have a lot more traffic. I have found that the key to running a successful website is making sure the visitors you are getting are interested in your niche. There is a company that you can get targeted visitors from and they let you try their service for free for 7 days. I managed to get over 300 targeted visitors to day to my site. Visit them here: http://9n3.us/7uza
May 16, 2015 at 2:06 am
Hi my name is Jacqueline and I just wanted to drop you a quick note here instead of calling you. I discovered your Using Untested Drugs On Africans | EbolaAlert Blog page and noticed you could have a lot more hits. I have found that the key to running a successful website is making sure the visitors you are getting are interested in your subject matter. There is a company that you can get targeted traffic from and they let you try the service for free for 7 days. I managed to get over 300 targeted visitors to day to my site. Visit them here: http://zoy.bz/4nn
July 27, 2015 at 3:25 pm
I like looking through an article that can make men and
women think. Also, thanks for permitting me to comment!
July 30, 2015 at 2:42 pm
Great work! This is the type of info that are supposed to be
shared around the web. Disgrace on the search engines for now not positioning
this post higher! Come on over and visit my web site
. Thank you =)